Home » Energy Conservation » Signet Eco Tec Power
Sulphation Causes Early Battery Death
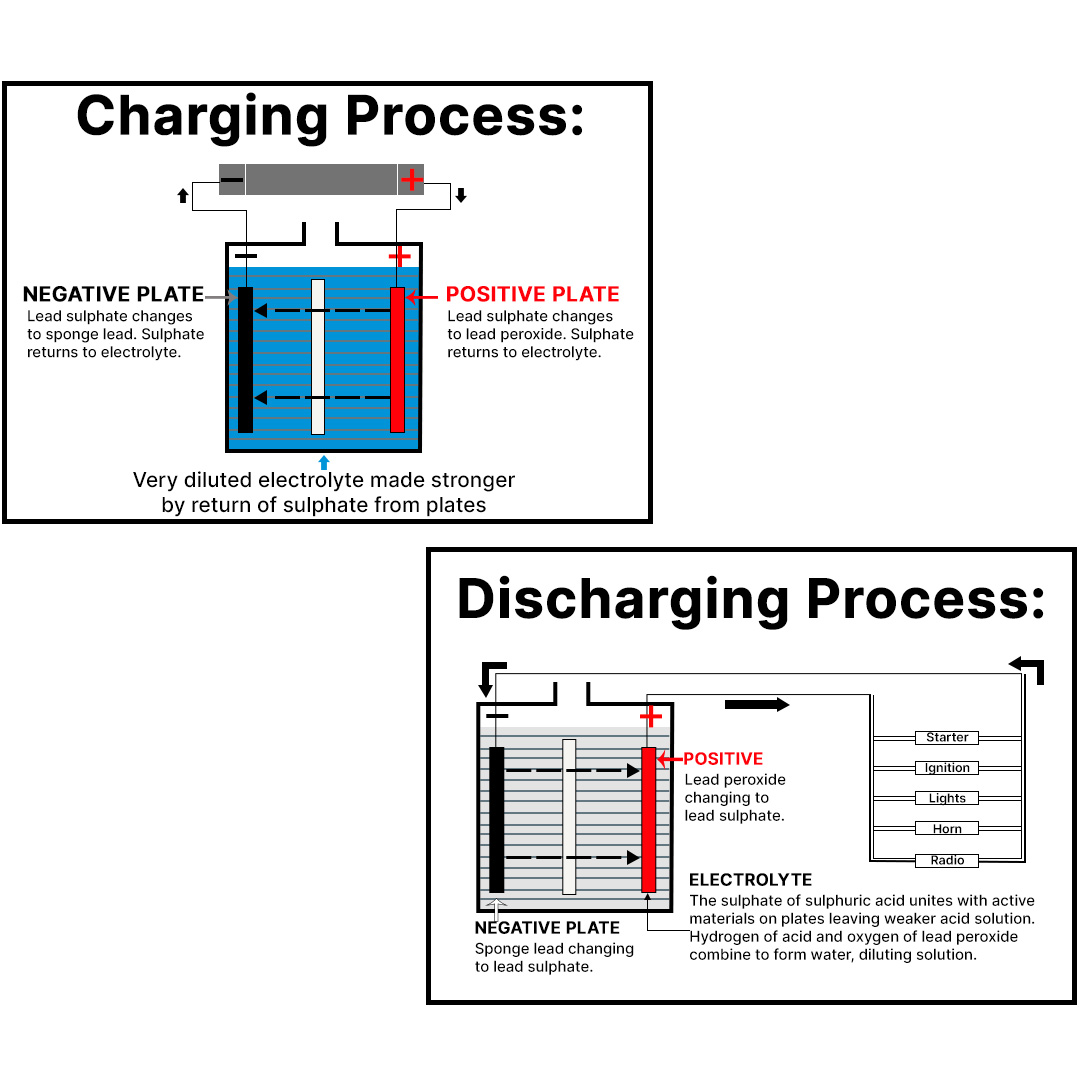
Research studies show ETP minimizes the accumulation and crystallization of sulphate ions. These ions (SO4), existing in the electrolyte (dilute sulfuric acid), become attached to the electrodes when discharged (the specific gravity decreases) and return to the solution when charged (the specific gravity increases).This is part of normal operations.
However, as this cycle is repeated, sulphate ions that are not actually involved in charging and discharging accumulate at the electrodes and crystallize, thus preventing full charging and discharging.
The product of this accumulation and crystallization is called “sulphation.” This is what causes battery performance to first deteriorate and then die — years earlier than necessary.
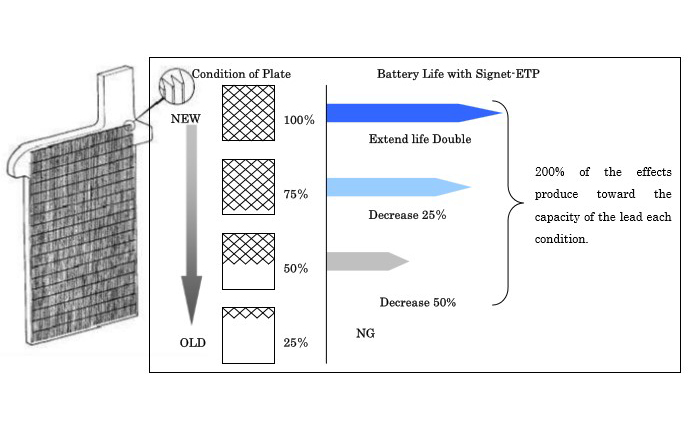
Scientific evidence as well as field tests were run on trucks, fork lifts, buses, and golf cars, etc. has shown that ETP extends battery life by preventing sulphation.
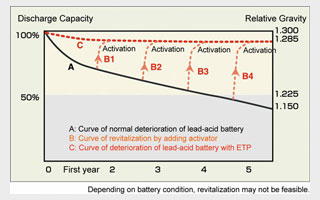
Relative Gravity Change
This relative gravity, which must increase (when charged) and decrease when discharged), is a key indicator of a lead-acid battery’s health. The chart shows the normal eterioration of a lead-acid battery both with and without ETP.
A satisfactory relative gravity at 20oC is 1.280. A difference in specific gravity of just 0.03 can make as much as a 20% difference in the discharge capacity. By adding ETP, the relative gravity will recover to 1.280 or greater in six months to one year.
Results By Type Of Battery
Field tests by the ITE Battery Research Institute were conducted on top quality batteries and inexpensive regular batteries — both with and without ETP.
The top quality battery maintained a high discharge capacity for 10 cycles, with or without ETP. After that, it deteriorated rapidly without ETP and slowly increased capacity with ETP.
The inexpensive battery showed immediate and rapid decreases in discharge capacity without ETP. With ETP the capacity increased slightly then decreased slowly.
ETP Technical Document
About The Degradation Factor Of The Lead Acid Battery
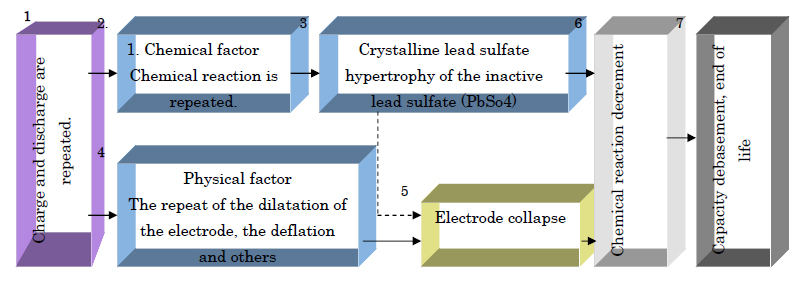
The major cause of the degradation of the lead acid battery is in the positive plate and the negative plate, and degradation advances it in relation to each other with both as well.
It is as follows when the general movement which degradation advances is put in order.
Chemical Factor
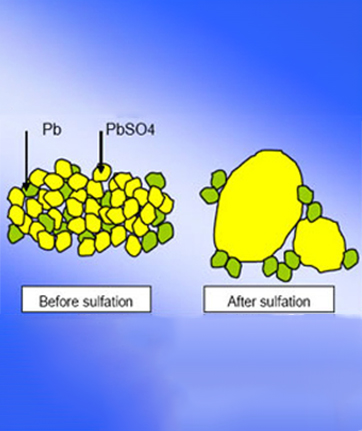
When Lead Acid Battery discharges, lead suphate (PbSo4) covers both the positive & the negative electrode. With time the crystallisation occurs in the pore of the electrode, capacity decreases because lead sulphate is a semi conductor & due due to crystallisation internal resistance increases consequently.
The solution decreases in density delivering a lowered battery output, this trend grows when the battery is left to discharge for long.
Physical Factor
Explanation of how chemical reaction decreases on repeat of charge and discharge phenomenon.
1. The collapse of the positive plate active material.
» Positive plate Erosion of the active material. The adhesion of the active material due to oxidation weakens the grid.
» Negative plate The degradation of the active material. Antimony (Sb) separates, and a micro-cell phenomenon happens due to the oxidation of the grid.
2. The exposure of the lead plate to the electrolyte decreases.
3. The swell up of the cap and the swell up of the compound.
Effect Of Signet-ETP
The addition of ETP is absorbed first by the electrode.
The lead sulphate (PbSo4) moleculewhich has bonded to the electrode at the time of discharging is detached;
this molecule polymer of ETP controls the lead sulphate crystallization.
In other words, the active surface of the electrode material will be secured. Moreover, it inhibits sulphation easily, and the growth of lead sulphate is reduced at the time of the charging.
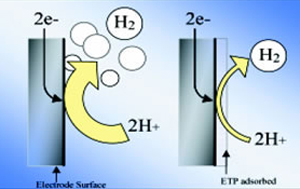
Absorbed on the negative electrode, ETP will inhibit the declining effect of hydrogen over voltage (the difference of the electrode potential when the hydrogen occurrence takes place) too. That effect will be lost by decomposing the oxidation of the ETP macro-molecule polymer gradually. That period is one, two years as a standard.
Excellent synergetic effect is shown which is greater than or equal to the cause of chemical degradation. The most important thing is keeping proper maintenance of the dedicated regenerated charger (a big current pulse charge) to achieve the activation of the lead acid battery by ETP.